Orbone® Cell and Tissue Bank Technology Platform
Making a Difference with Quality and Safety
A combination of common methods, chemical solvents, heat and oxidizing agents are used to clean, decellularize and sterilize tissue. In most processes, the use of solvents such as chloroform or acetone is necessary to defeat bone or decellularize tissue. The presence of oil, dermis or cells prevents tissue wetting, thereby reducing the activity of aqueous oxidizers.
Traditional tissue processing techniques rely heavily on chemical and biological agents. These agents adversely affect the natural, structural and mechanical properties of grafts. Chemicals cannot sufficiently penetrate deep into bone and soft tissue. As a result, the risk of toxic residues and immunological reactions, as well as processing efficiency, is reduced.
Orbone® Cell and Tissue Bank’s eCOO® scCO2 Technology, on the other hand, is the ideal method for cleaning, decellularization, virus inactivation, sterilization and inoculation while preserving the specific properties of the original tissues, depending on the nature of the raw tissue.
This unique process overcomes the shortcomings of tissues produced by conventional techniques, increasing sterilization, safety and regenerative properties, keeping the patient and patient safety as the highest priority. It accelerates the recovery of the patient and help them to gain their normal life in a very short time.
eCOO® Supercrit Technology
Besides viral inactivation, this process additionally removes fats, cell debris and marrow proteins. As the result, the organo-mineral structure of bone tissue which consists of apatite carburized in bone is perfectly preserved.
Various applications have been developed to date to devitalize bone tissues. These usually contain solvents for oxidative treatment of oils. In traditional methods, solvent (chloroform or acetone) is the main ingredient to dissolve the fat in the marrow cavity that contains adipose fat cells. The presence of large amounts of lipids inhibits aqueous oxidants, reduce the moisturizing ability of the tissues. Since diffusion in bone tissue is limited, the volume of bone fragments can be treated less effectively.
In addition, the use of degreasing solvents can cause unwanted toxic residues. Orbone® bone tissues are exposed to high pressure using eCOO®scCO2. As a result of this process, CO2 comes to “supercritical” phase, a phase between liquid and a gas. Regardless of the volume of the tissues, after transforming into the supercritical state, CO2 shows a solvent character, ensuring the diffusion of lipids from the tissues. Being a natural substance, eCOO® scCO2 leaves no toxic residue.
Orbone®’s production technology, eCOO® scCO2, is licensed and registered by the Dutch Ministry of Health according to European Directives (2004/23EC, 2006/86EC, 2006/17EC).
Orbone® human cells and tissues are meticulously produced and licensed by the Cell and Tissue Bank by the Turkish Republic Ministry of Health, are produced by applying the quality and safety requirements of the European directives.

Orbone® eCOO® scCO2 Technology provides an effective platform for cleaning and sterilizing tissues. Studies have shown that the use of eCOO® scCO2 minimally affects the structural integrity of tissue grafts compared to conventional processing techniques. eCOO® scCO2 therapy also removes the medullary tissues responsible for the adverse immune response. In addition to the eCOO® scCO2 treatment step, the eCOO® Clean process includes a viral inactivation step.
The combination of these two techniques has been shown to result in a 12-fold reduction in microbial load in processed grafts. Successful removal of cellular and medullary materials and inactivation of viruses is the most important step in providing better quality and cleaner grafts for patient outcomes.
Non-sterile cell-free tissues after cleaning from cell and lipid residues can also be sterilized using the technology platform’s eCOO® Ster process. The resulting sterile tissue can be freeze-dried or fresh-frozen. Alternatively, non-sterile cell-free tissue material can be first freeze-dried prior to final sterilization by irradiation technologies.
One of the most distinctive features of Orbone® eCOO® scCO2 Technology is that it can pass through solids and dissolve materials such as a liquid thanks to its gaseous structure. By controlling or regulating the pressure and temperature, the density or solvent strength of eCOO® scCO2 can be varied to simulate a wide variety of organic solvents. This dissolving power is applied to obtain, extract and sterilize a wide variety of sensitive materials. The low temperature of the process and the stability of CO2 also allow compounds to be removed without damaging or denaturation of allograft tissues.
Due to these properties, the eCOO® scCO2 Technology can greatly reduce the presence of microorganisms, thereby thoroughly cleansing the tissues, while also meeting the sterility assurance level of 10-6 (SAL6) for all types of microorganisms.
Viral Inactivation and Safety
A viral inactivation study conducted by Institute Pasteur Texcell in Paris in 1995 demonstrated the effectiveness of this process over viral inactivation. The results obtained are superior to the safety level recommended by the European guidelines (6 log reduction versus 14 log reduction). The results have been confirmed by a study by Sanquin Pharmaceutical Services on behalf of eCOO® Technology.
Sterilization
When using eCOO® Technology to introduce and extract bactericidal sterilizers into tissue like peri-acetate acid, the process renders tissue sterile by inactivating a wide range of bacteria, including Bacillus.
Biogenesis and Osseointegration
Allografts treated with Orbone® eCOO® Technology lead to a much faster osseointegration compared to untreated allografts. Treatments based on Orbone® eCOO® Technology do not change the biomechanical properties of the tissue.
Clinical Evidence
The processed Orbone® eCOO® grafts have proven to have superior biochemical composition, biocompatibility, tolerance and biofunctional properties compared to grafts processed using conventional methods.
State-of-the-art tissue products that are clean and sterile (SAL6) without compromising on structural integrity
• Excellent biocompatibility and advanced regenerative properties
• Adaptable to meet tissue specific demands
• It does not leave hazardous material residues
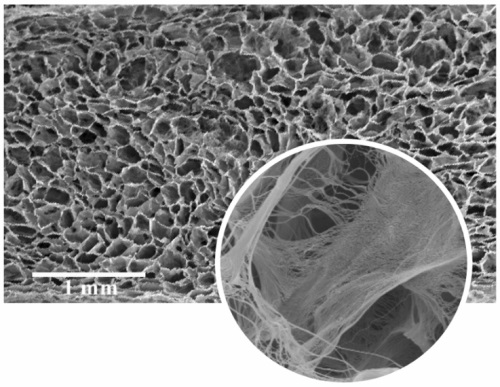
SEM images confirm that eCOO® scCO2 minimally affects the extracellular matrix while removing medullary tissues.
The Orbone® Tissue Bank eCOO® Technology Platform encompasses cutting-edge technology handles for cleaning, sterilizing and inoculating biological grafts using a breakthrough solution based on supercritical carbon dioxide scCO2. The eCOO® scCO2 extraction process is highly effective in inactivating viruses and provides superior inactivation when compared to tissues obtained by conventional methods.